![Ni(CO)4 has a tetrahedral geometry whereas [Ni(CN)4]4– has square planar geometry. Why? from Chemistry Coordination Compounds Class 12 Jharkhand Board Ni(CO)4 has a tetrahedral geometry whereas [Ni(CN)4]4– has square planar geometry. Why? from Chemistry Coordination Compounds Class 12 Jharkhand Board](https://www.zigya.com/application/uploads/images/chen12070385_571483de917be.png?t=1460962273442)
Ni(CO)4 has a tetrahedral geometry whereas [Ni(CN)4]4– has square planar geometry. Why? from Chemistry Coordination Compounds Class 12 Jharkhand Board
Write the IUPAC nomenclature of the given complex along with its hybridisation and structure K2[Cr(NO)(NH3)(CN)4], μ = 1.73. - Sarthaks eConnect | Largest Online Education Community
Supporting Information for “Relevant electronic interactions related to the coordination chemistry of tetracyanometallates. An
![Predict the number of unpaired electrons in the square planar [Pt(CN)4,]2– ion. from Chemistry Coordination Compounds Class 12 Maharashtra Board Predict the number of unpaired electrons in the square planar [Pt(CN)4,]2– ion. from Chemistry Coordination Compounds Class 12 Maharashtra Board](https://www.zigya.com/application/uploads/images/chen12070040_57074e44aa50d.png?t=1460096581824)
Predict the number of unpaired electrons in the square planar [Pt(CN)4,]2– ion. from Chemistry Coordination Compounds Class 12 Maharashtra Board
![According to the valence bond theory, the hybridization of central metal atom is dsp2for which of the following compounds?a)Na2[NiCl4]b)NiCl2.6H2Oc) K2[Ni(CN)4]d)[Ni(CO)4]Correct answer is option 'C'. Can you explain this answer? | EduRev JEE Question According to the valence bond theory, the hybridization of central metal atom is dsp2for which of the following compounds?a)Na2[NiCl4]b)NiCl2.6H2Oc) K2[Ni(CN)4]d)[Ni(CO)4]Correct answer is option 'C'. Can you explain this answer? | EduRev JEE Question](https://edurev.gumlet.io/ApplicationImages/Temp/003bd7dd-46f9-4901-8626-8f092d259fd3_lg.jpg)
According to the valence bond theory, the hybridization of central metal atom is dsp2for which of the following compounds?a)Na2[NiCl4]b)NiCl2.6H2Oc) K2[Ni(CN)4]d)[Ni(CO)4]Correct answer is option 'C'. Can you explain this answer? | EduRev JEE Question
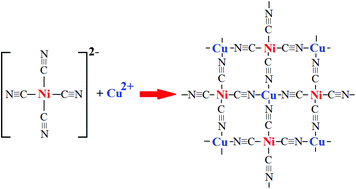
Chemistry and structure by design: ordered CuNi(CN)4 sheets with copper(ii) in a square-planar environment - Dalton Transactions (RSC Publishing)
 - Brainly.in inter-halogen compounds.निम्नलिखित उपसहसंयोजी यौगिकों के IUPAC नाम नि(i) K2[ Ni(CN)4](ii) - Brainly.in](https://hi-static.z-dn.net/files/da4/7d0a4460d907e49ec040a6dbfaa88371.jpg)

![Calculate the oxidation number of Ni in K(2)[Ni(CN)(4)] . Calculate the oxidation number of Ni in K(2)[Ni(CN)(4)] .](https://d10lpgp6xz60nq.cloudfront.net/ss/web/265541.jpg)
![Calculate the oxidation number of Ni in `K_(2)[Ni(CN)_(4)]` . - YouTube Calculate the oxidation number of Ni in `K_(2)[Ni(CN)_(4)]` . - YouTube](https://i.ytimg.com/vi/lE5DlvdO6mo/maxresdefault.jpg)
![Give the IUPAC name of : (i) K2[Ni(CN)4] ii) [CoCL2(NH3)4]Cl Give the IUPAC name of : (i) K2[Ni(CN)4] ii) [CoCL2(NH3)4]Cl](https://d10lpgp6xz60nq.cloudfront.net/ss/web/790121.jpg)
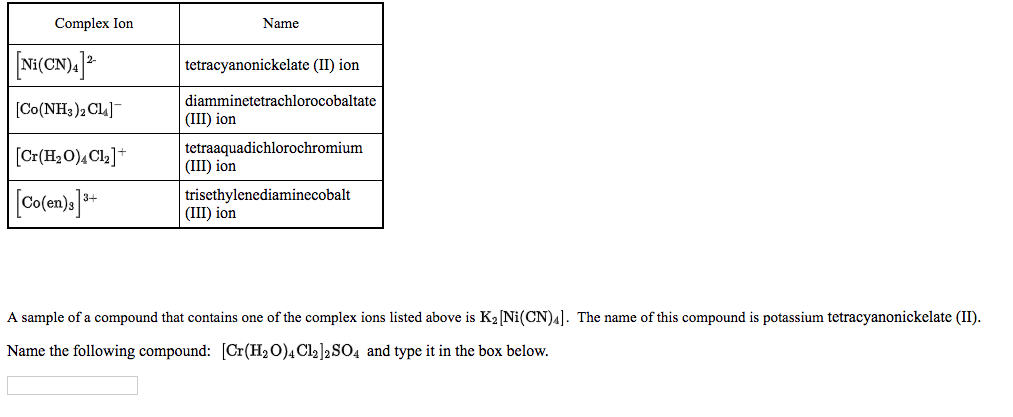
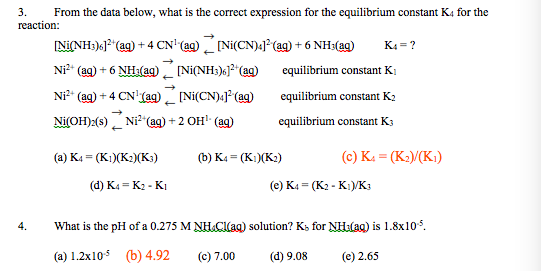
![Hybridization and geometry of [Ni(CN)4]^2 - are: Hybridization and geometry of [Ni(CN)4]^2 - are:](https://haygot.s3.amazonaws.com/questions/1453910_739662_ans_1b88a50e6b3142878d714df46d9472b7.png)
![Oxidation number of Ni in. K2[Ni(CN)6] - Brainly.in Oxidation number of Ni in. K2[Ni(CN)6] - Brainly.in](https://hi-static.z-dn.net/files/d50/f32591cdafb7d03c82862669f643a135.jpg)
![What is the hybridisation for [Ni(CN) 4] 2-? - Quora What is the hybridisation for [Ni(CN) 4] 2-? - Quora](https://qph.cf2.quoracdn.net/main-qimg-20bb619759136ec1b4419262dd5a28a0.webp)
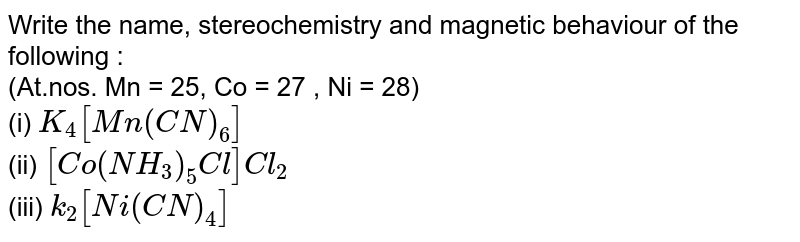
![SOLVED: write the name of K2[Ni(CN)4] and how many unpaired electrons in complex? SOLVED: write the name of K2[Ni(CN)4] and how many unpaired electrons in complex?](https://cdn.numerade.com/ask_previews/2ec2b21e-ae21-4f6d-94de-26e4b2ab7c09_large.jpg)
![The hybridization of central metal ion in K2[Ni(CN)4] and K2[NiCl4] are respectively The hybridization of central metal ion in K2[Ni(CN)4] and K2[NiCl4] are respectively](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/647498335_web.png)
![Oxidation number of Ni in. K2[Ni(CN)6] - Brainly.in Oxidation number of Ni in. K2[Ni(CN)6] - Brainly.in](https://hi-static.z-dn.net/files/ddc/f5fd5d041b43dc96515c7c77cac9509f.jpg)

![Ni(CO)4, [Ni(CN)4]2-, [NiCl4]2-Structure-Hybridization-VBT-IIT JEE NEET SAT NCERT CBSE - YouTube Ni(CO)4, [Ni(CN)4]2-, [NiCl4]2-Structure-Hybridization-VBT-IIT JEE NEET SAT NCERT CBSE - YouTube](https://i.ytimg.com/vi/r_C4yyTUSjM/mqdefault.jpg)
![The IUPAC name of K2[Ni(CN)4] is | Filo The IUPAC name of K2[Ni(CN)4] is | Filo](https://askfilo.com/_next/image?url=https%3A%2F%2Fstorage.googleapis.com%2Ffilo-classroom-notes%2Fthumb_classroom_27270786_RJEY6.jpeg&w=1920&q=75)

![K2[Ni(CN)4] = Ni + 2KCN + C2N2 | Chemical Equation K2[Ni(CN)4] = Ni + 2KCN + C2N2 | Chemical Equation](https://chemicalequationbalance.com/uploads/images/20190506030723000000-tai_xuong.jpg)
